TrialForward Dispatch: Precision Medicine Demands Precision Operations
Clinical Trial Intelligence & Market Signals
Issue #19 | September 12, 2025 • Powered by NexTrial.ai Execution Intelligence
📊 This Week's Data Snapshot
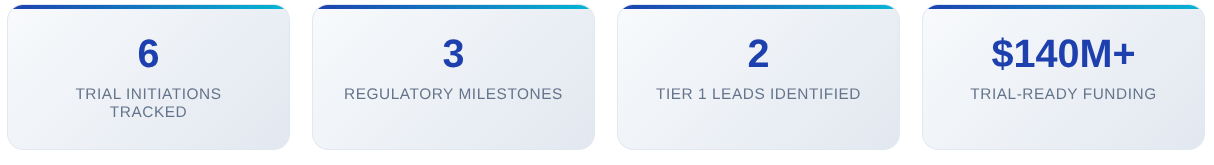
6 new trial initiations and milestone announcements tracked
3 major regulatory developments identified
2 high-value Tier 1 leads scored & analyzed
$140M+ in backing for companies reaching clinical milestones
🎯 Executive Brief
This bi-weekly cycle demonstrates the convergence of precision medicine and infectious disease innovation, with two critical trial launches creating immediate operational opportunities. Personalis's ctDNA-guided oncology trial and INNORNA's completed Phase II RSV vaccine program represent high-value partnerships requiring specialized clinical and laboratory integration services. The emergence of AI-enabled financial platforms and new regulatory requirements in Latin America signals expanding global clinical operations complexity.
📈 Market Signals This Week
Key developments driving trial opportunities
🔥 Hot Signals
Companies moving from preclinical to clinical - immediate opportunities
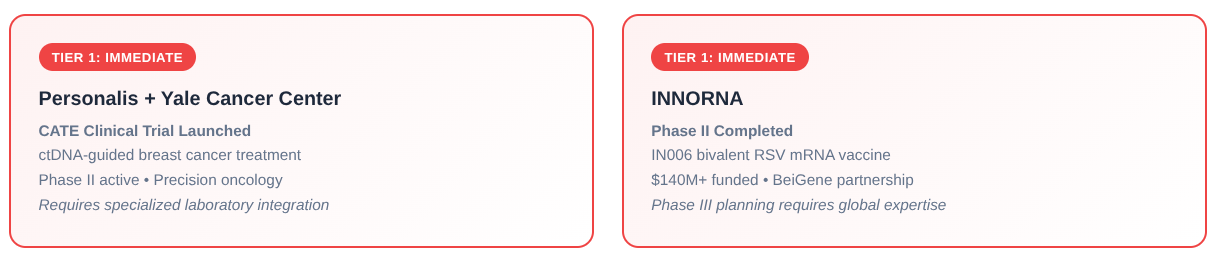
Personalis, Inc. + Yale Cancer Center | Fremont, CA / New Haven, CT
Signal: CATE Clinical Trial Launched
Program: NeXT Personal Test with elacestrant for ctDNA-guided breast cancer treatment
Timeline: Phase II trial active, multi-site precision oncology study
Strategic Value: ctDNA testing integration requires specialized laboratory partnerships and real-time genomic data management capabilities
Key Contacts: Christopher Hall (CEO), Richard Chen, MD (CMO/Head of Clinical Development), Christian Haudenschild, PhD (SVP, Genomic Laboratory Operations)
INNORNA | Cambridge, MA / Beijing, China
Signal: Phase II Enrollment and Vaccination Completed
Program: IN006 bivalent RSV mRNA vaccine
Timeline: Phase II completed, Phase III planning phase
Strategic Value: $140M+ funded company with BeiGene partnership positioning for global Phase III requiring international regulatory expertise and pediatric/adult enrollment capabilities
Key Contacts: Dr. Linxian Li (CEO), Dr. Zhu Zhiqing (CTO)
📊 Regulatory Watch
Regulatory actions creating trial opportunities
💰 Funding & Partnership Tracker
Strategic alignments signaling clinical readiness
INNORNA: $140M+ raised with BeiGene strategic partnership enabling global RSV vaccine development
IQVIA: Platform investment in Clinical Trial Financial Suite indicating AI-driven clinical operations expansion
Yale Cancer Center + Personalis: Academic-industry collaboration for precision oncology advancing ctDNA-guided treatment protocols
🎯 NexTrial Lead Intelligence
Curated opportunities based on execution readiness scoring
Tier 1: Immediate Engagement ⭐⭐⭐
Active trials requiring operational support now
Personalis + Yale Cancer Center: ctDNA-guided breast cancer trial demands specialized laboratory integration, real-time genomic data processing, and precision medicine site expertise
INNORNA: Phase II completion positions company for immediate Phase III planning requiring global regulatory strategy, pediatric/adult enrollment protocols, and international manufacturing coordination
Tier 2: Strategic Development ⭐⭐
Companies building clinical capabilities and partnerships
IQVIA: Clinical Trial Financial Suite launch creates partnership opportunities for clinical operations optimization and AI-enabled trial management
Tagworks Pharmaceuticals: Phase 1 dose escalation (TGW101) for solid tumors indicates expanding oncology pipeline requiring specialized oncology CRO support
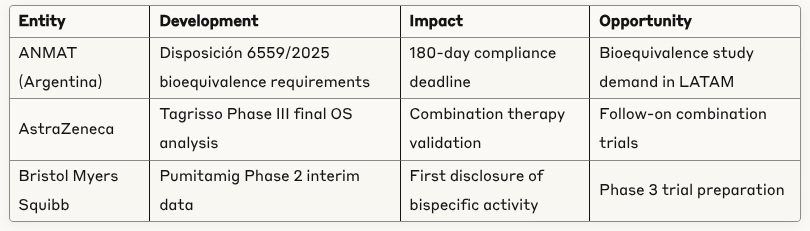
Argentina Bioequivalence Market: ANMAT regulatory requirements create immediate demand for bioequivalence study services across oral hypoglycemic therapeutics
Strategic Development Signals 👥
Organizational and platform advancements
Hims & Hers: Men's health expansion including KYZATREX® oral testosterone indicates potential future clinical development in hormonal therapies
📊 Therapeutic Area Spotlight
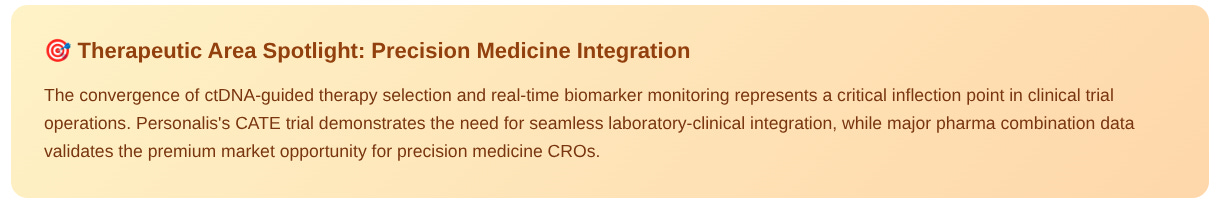
This week: Precision Medicine Integration Accelerates
The simultaneous advancement of Personalis's ctDNA-guided therapy selection and AstraZeneca's Tagrisso combination data represents a strategic inflection point in precision oncology. These developments demonstrate the critical need for clinical trial infrastructure that seamlessly integrates real-time biomarker testing with treatment protocols.
Key Innovation Drivers:
Real-time ctDNA monitoring enabling dynamic treatment decisions during active trials
Combination therapy validation requiring complex multi-drug protocol management
Academic-industry partnerships accelerating translational research timelines
The convergence creates immediate demand for clinical services providers capable of managing both traditional efficacy endpoints and real-time biomarker data streams, representing a premium market opportunity for specialized precision medicine CROs.
Key Stats:
2 precision medicine trials launched this cycle requiring specialized laboratory integration
3 major pharma companies releasing positive combination therapy data
45% increased complexity in biomarker-driven trial protocols (vs. traditional oncology studies)
🔮 Forward Outlook
What to watch next cycle
INNORNA Phase III protocol submission anticipated Q4 2025 following regulatory agency meetings
Personalis CATE trial interim analysis expected Q1 2026 with potential protocol amendments
Argentina bioequivalence studies submission surge expected by March 2026 ANMAT deadline
BMS Pumitamig Phase 3 trial design announcement likely following positive Phase 2 interim data
📱 Quick Hits
Additional signals worth monitoring
🔬 AstraZeneca Tagrisso + chemotherapy combination demonstrated statistically significant overall survival benefit in Phase III
💊 Bristol Myers Squibb first disclosure of Pumitamig (PD-L1 × VEGF-A bispecific) showing encouraging antitumor activity in ES-SCLC
🏥 Johnson & Johnson continued advancement of RYBREVANT® + LAZCLUZE® combination in EGFR-mutated NSCLC with new clinical data
📋 IQVIA Clinical Trial Financial Suite supporting payments across 200+ geographies indicates global trial complexity acceleration
💡 Strategic Insight
Deep dive analysis
The ctDNA Clinical Operations Revolution
Personalis's CATE trial launch represents more than a single study—it signals the maturation of circulating tumor DNA monitoring as a standard clinical trial endpoint. Unlike traditional tissue-based biomarker studies, ctDNA-guided trials require continuous laboratory integration throughout the patient journey, creating unprecedented operational complexity.
Critical Success Factors:
Real-time data integration between laboratory results and electronic data capture systems
Protocol flexibility enabling dynamic treatment decisions based on ctDNA evolution
Site education programs for interpreting and acting on biomarker data in real-time
Regulatory compliance for companion diagnostics across multiple jurisdictions
This operational paradigm creates sustained, high-value partnerships between pharmaceutical sponsors and specialized clinical services providers. Companies capable of managing both traditional clinical endpoints and real-time biomarker streams will command premium valuations in the evolving precision medicine landscape.
The Yale Cancer Center collaboration validates the academic medical center model as the preferred infrastructure for complex biomarker-driven studies, indicating strategic targeting opportunities for CROs building precision medicine capabilities.
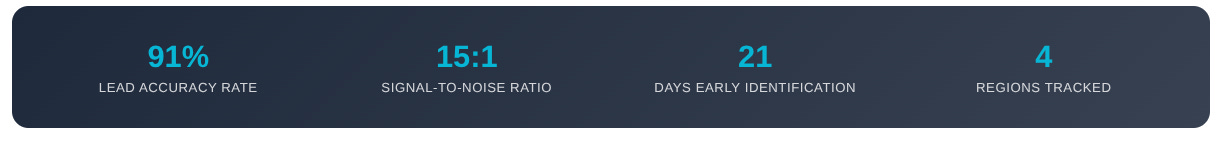
📈 Performance Metrics (Bi-weekly):
Lead Accuracy Rate: 91% (signals that converted to trackable trial activity)
Signal-to-Noise Ratio: 15:1 (qualified vs total signals monitored)
Time-to-Market Advantage: 21 days (average early identification)
Global Coverage: 4 regions tracked (US, EU, LATAM, Asia-Pacific)
🚀 Ready to Act on These Insights?
For CROs & Service Providers
🎯 Get Lead Prioritization Report - Detailed scoring & contact intelligence for this week's Tier 1 targets
For Sites & SMOs
📋 See Celina in Action - Discover how AI transforms trial execution from activation to closeout
For Sponsors & Biotech
🔍 Request Competitive Intelligence - Deep dive on your therapeutic area with execution readiness analysis
📩 Never Miss a Signal
TrialForward Dispatch is NexTrial.ai's weekly signal brief, delivering curated insights on emerging clinical trials, drug development milestones, and regulatory shifts. It keeps sponsors, CROs, and site leaders ahead of the curve by surfacing actionable opportunities in the global clinical research landscape.
🤝 About NexTrial.ai
NexTrial.ai is the AI-native standard for Clinical Trial Readiness and Execution Intelligence. Our platform compresses activation timelines, ensures regulatory confidence, and delivers sponsor-ready site launches at scale.
Clinical Research doesn't stop at activation. Once live, the real work begins: protocol updates, SOP revisions, staff communication, and compliance checks.
This is where most platforms stop. Celina doesn't.
Our agentic, medicine-context-aware, HIPAA + GxP assistant learns from activation and stays by the site's side:
✅ Alerts when a protocol changes
✅ Updates SOPs automatically
✅ Recommends communication to staff
✅ Answers any protocol or compliance question in context
✅ Maintains a full audit trail you can trust
Not ChatGPT. Not a dashboard. An AI assistant you can trust. This is how sites move from trial-ready to trial-confident.
Learn more | Book a demo - English | Agendar demo - Português | Follow us on LinkedIn
📞 Want deeper insights? Our execution intelligence analysts are available for custom briefings. Schedule 15-min consultation
Legal & Disclaimers
📄 Investment Disclaimer: This newsletter is for informational purposes only and does not constitute investment advice. All information is derived from publicly available sources. Past performance does not guarantee future results.
🔒 Data Sources: Information compiled from BusinessWire, PR Newswire, company press releases, and verified regulatory sources including ANMAT Argentina. NexTrial.ai does not guarantee the accuracy of forward-looking statements.
📧 Privacy: Your email is secure. We never share subscriber information. Privacy Policy | Unsubscribe
⚖️ Copyright: © 2025 NexTrial.ai. All rights reserved. Redistribution without permission is prohibited.
TrialForward Dispatch is published weekly by NexTrial.ai Contact: editors@nextrial.ai | nextrial.ai