Trial Forward Dispatch #13 — The Product Owner Build Pack
Inside the Build: Product, Process & AI Orchestration for Clinical Trial Activation How we turn the messy reality of activating clinical trial sites into an orchestrated product system that actually w
The Real Build Behind NexTrial
Here's what most people don't understand about building AI for clinical trials: The real build is not "AI."
It's Product System Design — where AI becomes one component of a larger operating model that actually works in the regulated, relationship-driven world of clinical research.
I asked ChatGPT to generate an image of what it feels like to be me, building this — with full honesty, openness, and vulnerability. What came back was this swirling orchestration of networks, business models, sprint boards, and complexity flowing around a founder who's somehow managing to smile through it all.
Not art. A mirror.
Because this is what it actually looks like to build regulated AI that moves the needle: Product Owner discipline meets clinical operations reality, wrapped in compliance architecture, delivered through change management.
Section 1: The Activation Problem (Through a Product Owner Lens)
Every clinical trial has the same bottleneck: Site activation takes 6-12 months when it should take 6-12 weeks.
Why? Fragmentation everywhere:
Protocol complexity that requires PhD-level interpretation
Regulatory requirements that vary by site, region, and indication
Resource planning that's more art than science
Communication loops that break down between sponsors, CROs, and sites
As a Product Owner, I frame this through Jobs To Be Done: Activation = Job #1. Everything else — enrollment, data collection, closeout — depends on getting sites activated faster and with higher confidence.
The job isn't just "make it faster." The job is:
"Help me confidently commit to a trial timeline, knowing I have the resources, training, and support to execute successfully."
That's a Product + Process + Trust Problem, not just a workflow problem.
Section 2: Product Architecture Approach
We start with Problem Framing Framework (PFF) — deconstructing complexity before we build anything:
Layer 1: Clinical Logic
Protocol requirements → Site capabilities mapping
Resource forecasting → Capacity planning
Training needs → Competency validation
Layer 2: Process Architecture
BPMN as Product Blueprint
Human-in-the-Loop (HIL) decision points
Agent handoff choreography
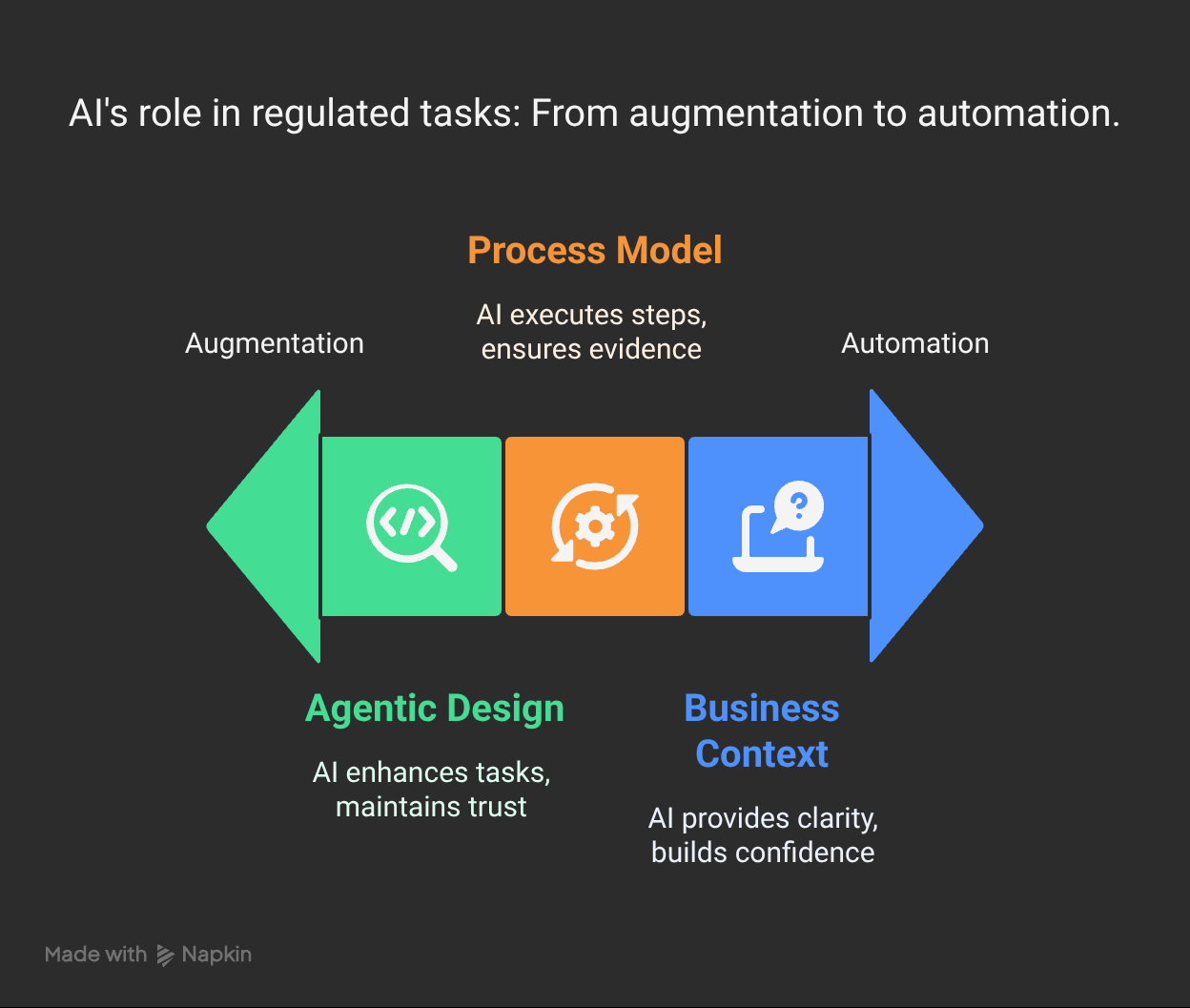
Layer 3: AI Agent Design
Explainable reasoning chains
Audit trail generation
Compliance-first prompt engineering
Product Ownership means mapping agent roles before building agents. Each AI component has:
Defined scope of authority
Clear escalation triggers
Human oversight requirements
Explainability standards
We're not building "smart automation." We're building augmented decision-making that clinical professionals can trust and auditors can validate.
Section 3: Product Operations Discipline
Building in healthcare means Product Ops isn't optional — it's survival.
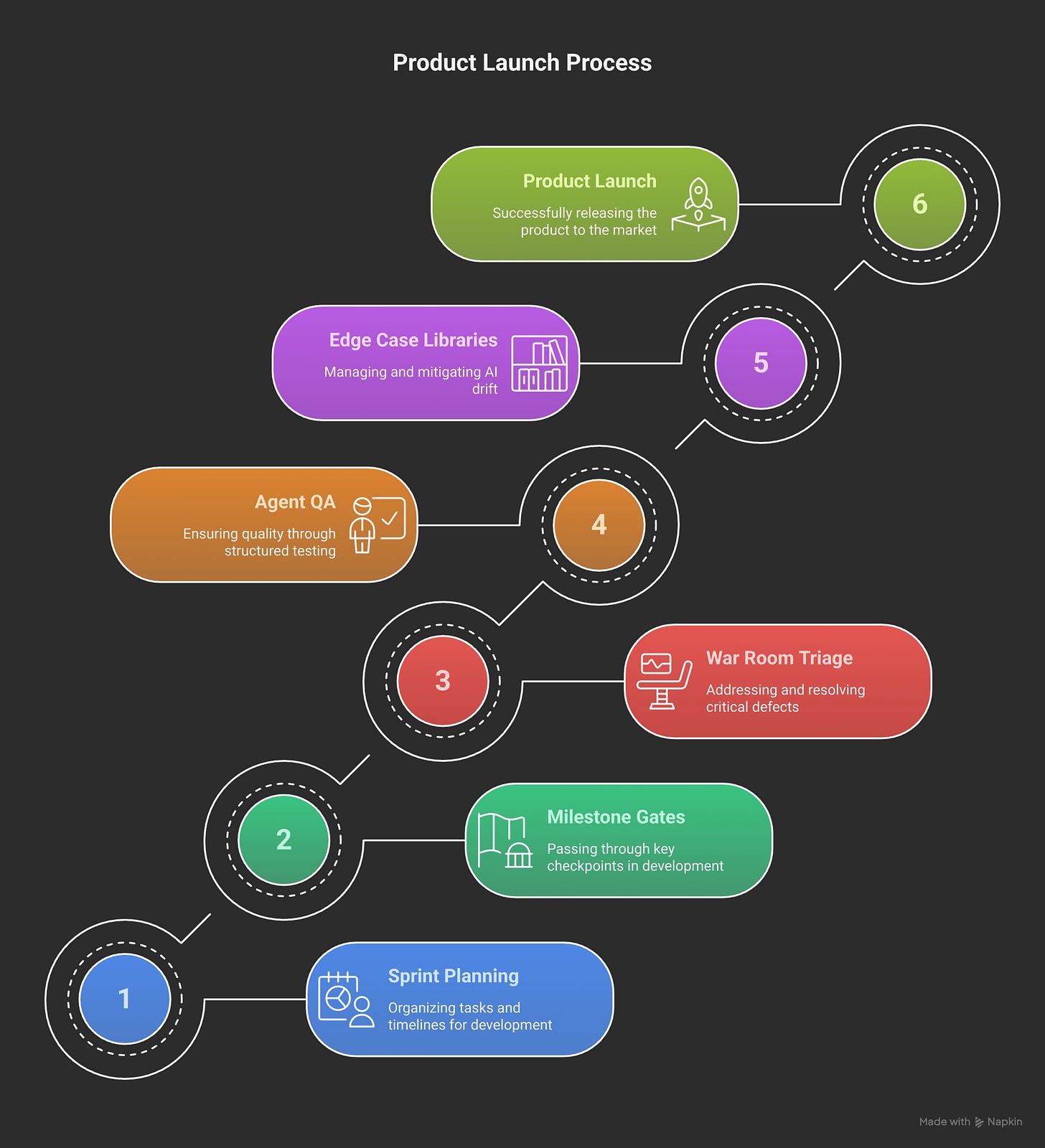
Sprint Planning Reality:
2-week sprints with clinical stakeholder validation every cycle
QA → PIT → SIT cycles embedded in every feature release
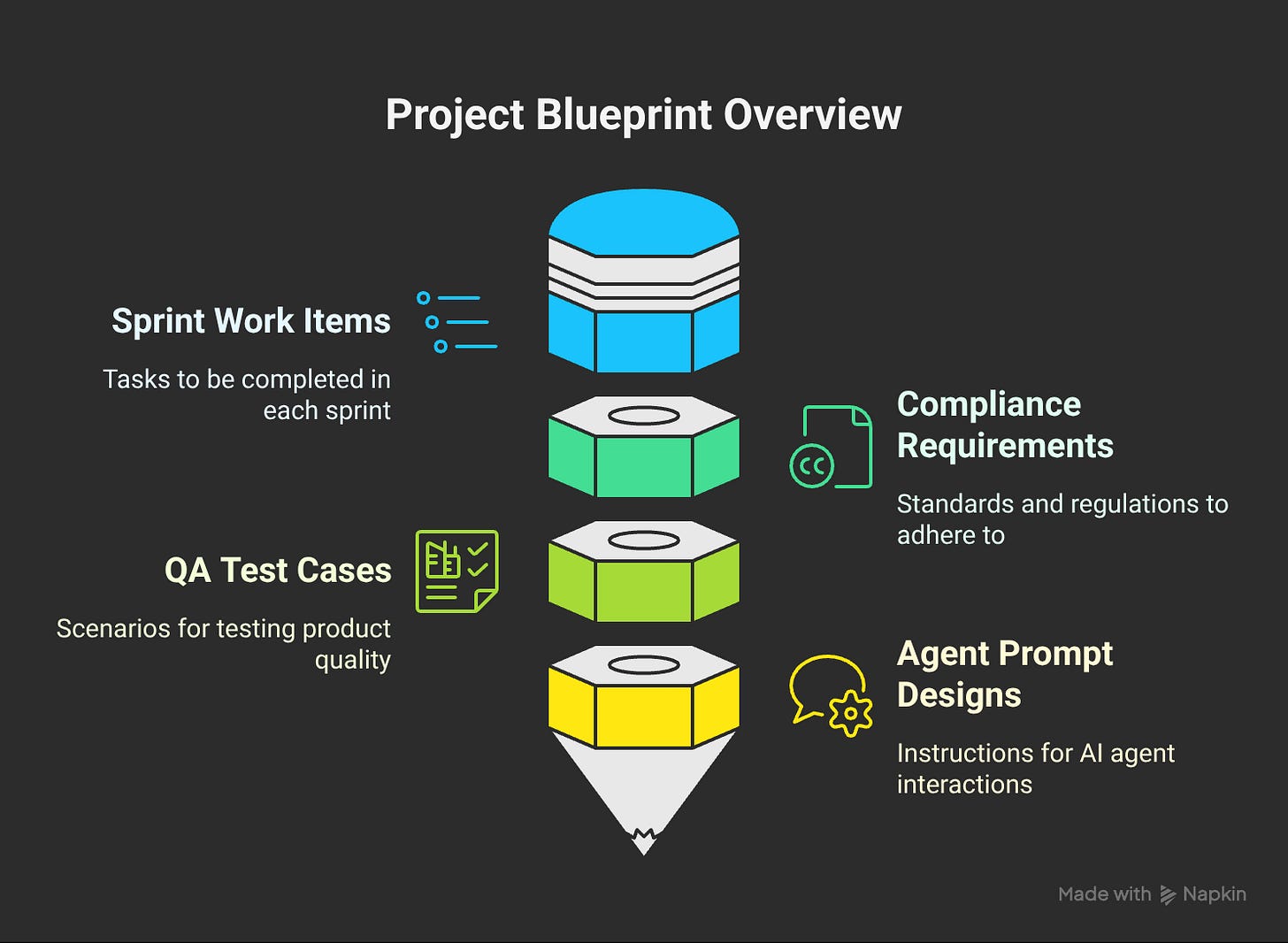
Agent Behavior Documentation as first-class product artifacts
Edge Case Handling designed upfront, not retrofitted
Our Prompt Library is a Product Artifact. Every prompt gets:
Version control
Performance metrics
Clinical review approval
Regression testing protocols
Daily Operations:
Morning standups: What broke overnight?
Afternoon war rooms: What's blocking go-live?
Weekly retrospectives: What compliance gaps did we miss?
Product Operations in clinical trials means operational discipline meets regulatory rigor. Every build decision cascades through compliance, training, and change management implications.
Section 4: Project Management Backbone
Gate Reviews drive everything:
FIT (Feasibility): Can we solve this problem?
PIT (Proof of Concept): Does our approach work?
SIT (System Integration): Can it work at scale?
Beta: Will clinical users adopt it?
Launch: Can we support it operationally?
We use Plane.so as our PMO Operating System because clinical trials need project management that's:
Milestone-based (not just task-based)
Stakeholder-centric (PIs, sites, sponsors, regulators)
Evidence-generating (every decision needs documentation)
Sprint Design Philosophy:
Week 1: Build + Internal QA
Week 2: Clinical stakeholder validation + iteration
Gate Review: Go/No-Go for next sprint
The difference between software PMO and clinical PMO? Every decision has regulatory implications, and every timeline has patient impact.
Section 5: Organizational Change Management (OCM) Integration
Here's what most HealthTech builders miss: Technology adoption in clinical research is fundamentally a change management problem.
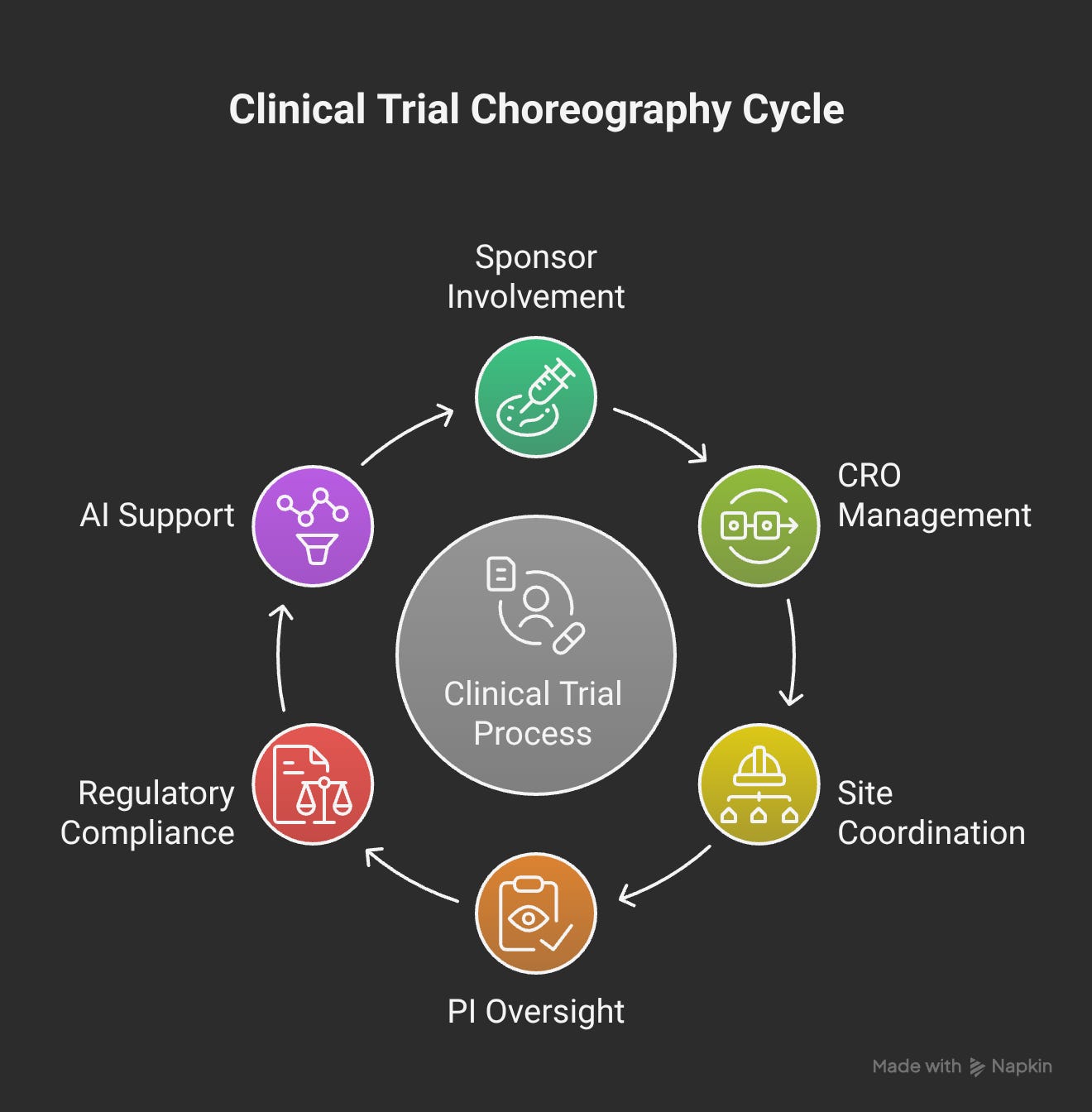
Stakeholder Alignment Reality:
Principal Investigators: Need confidence, not complexity
Site Coordinators: Need efficiency, not more work
Sponsors: Need predictability, not promises
CROs: Need scalability, not one-off solutions
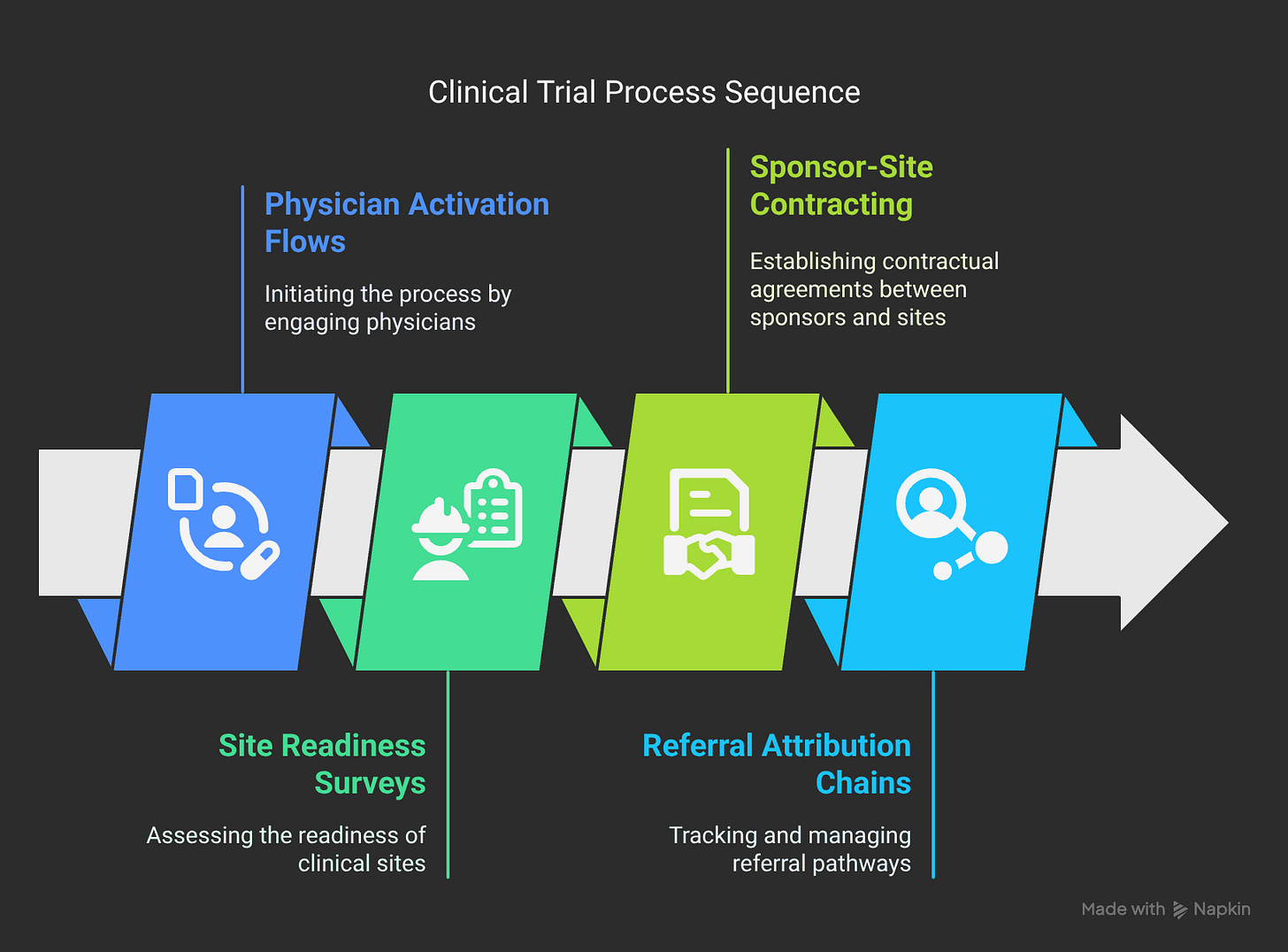
Physician Activation Journey Mapping:
Awareness: "What is this, and why should I care?"
Interest: "How does this solve my specific problems?"
Evaluation: "Can I trust this with my patients and my reputation?"
Trial: "Will this actually work in my environment?"
Adoption: "How do I make this part of my standard workflow?"
Advocacy: "How do I help improve this for other sites?"
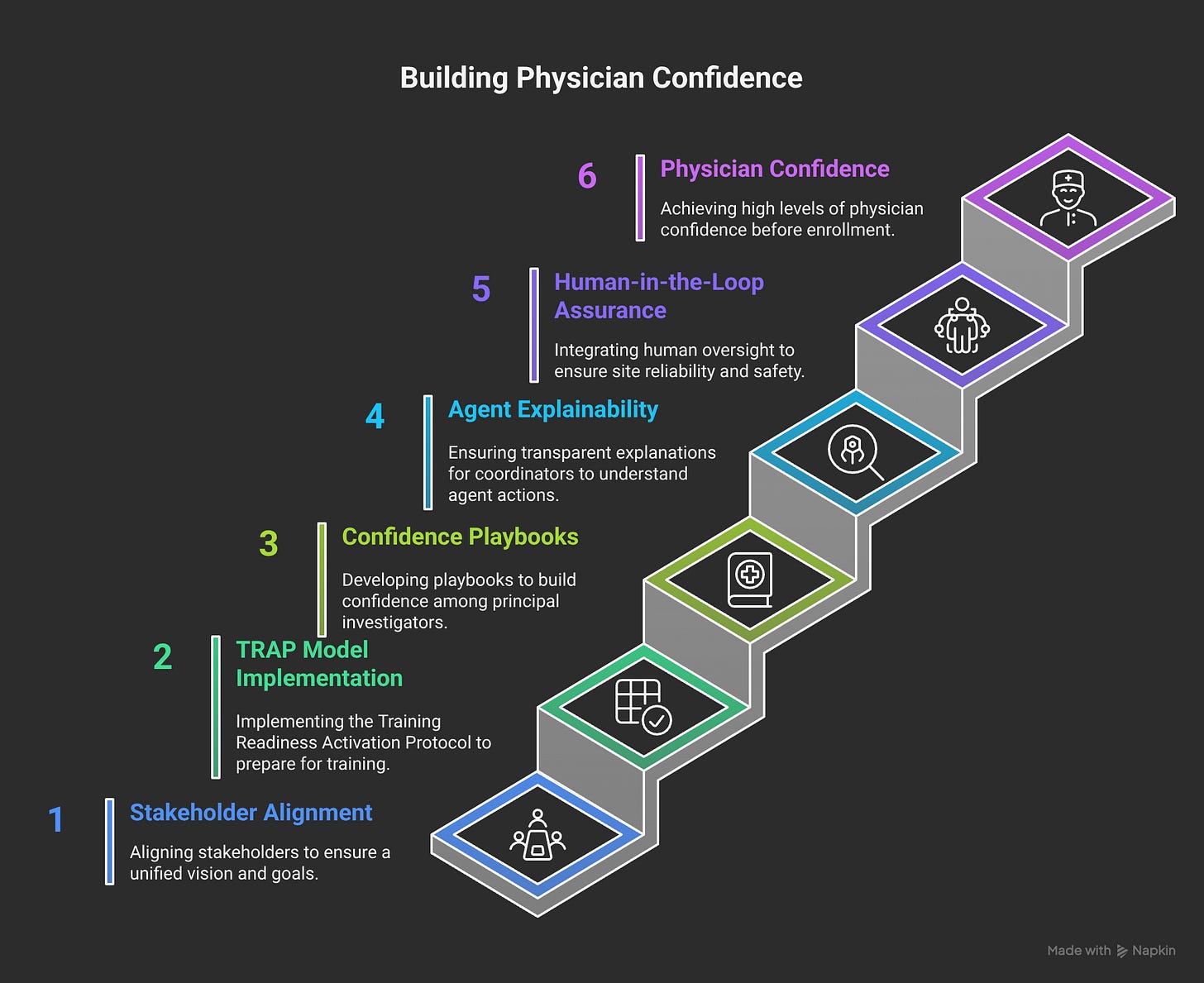
TRAP Model (Training Readiness Assessment Protocol):
Technical Readiness: Do they understand the system?
Resource Readiness: Do they have capacity to implement?
Authority Readiness: Do they have buy-in from leadership?
Process Readiness: Are their workflows compatible?
We embed Change Agents directly into Product Activation. Every beta site gets:
Dedicated implementation specialist
Weekly adoption metrics review
Direct feedback loop to product development
Success story documentation for future sites
Section 6: Compliance & Regulatory Architecture
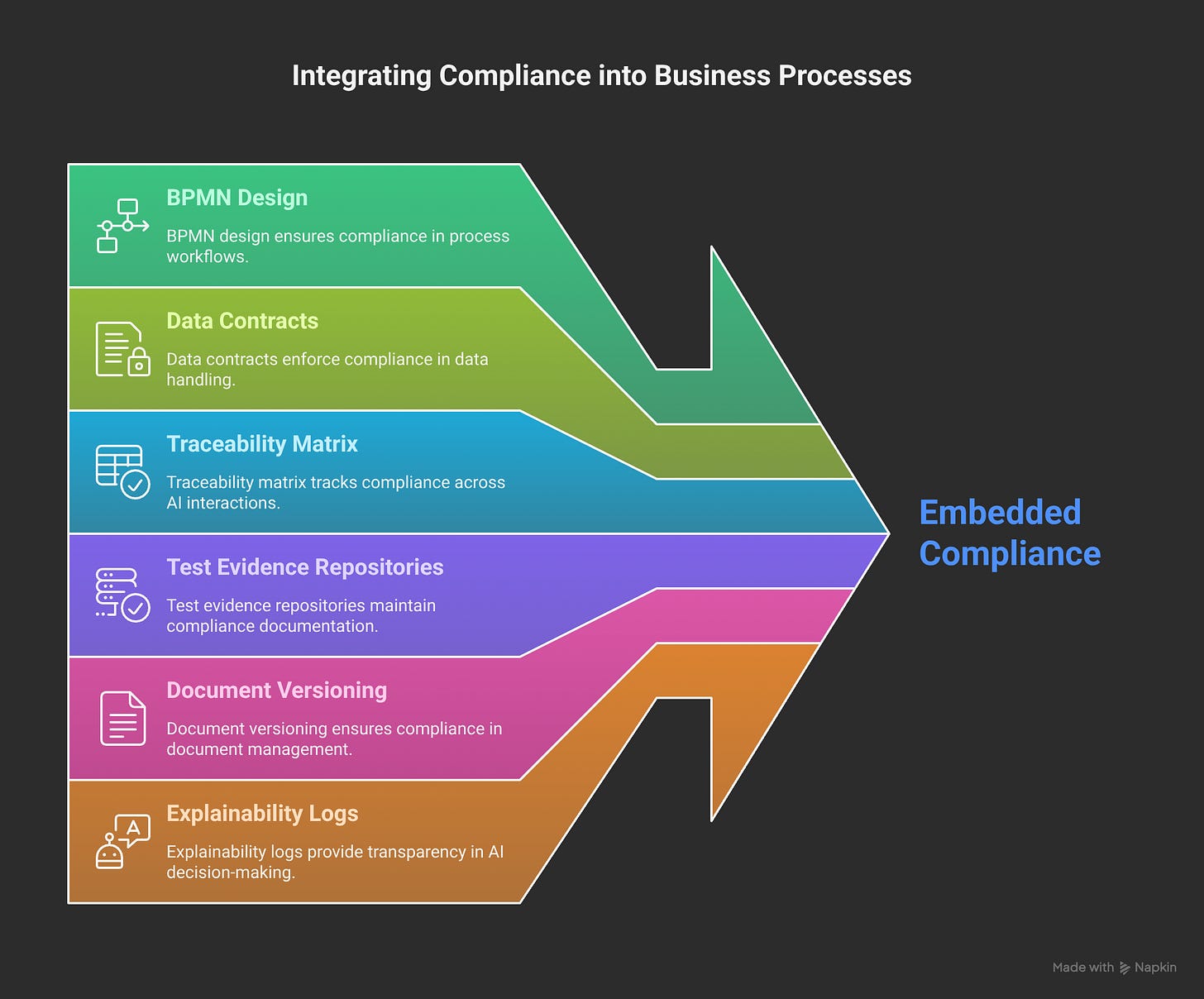
GCP, ICH, Data Contracts aren't constraints — they're product requirements.
Every feature gets designed with Traceability Matrix thinking:
Prompt → Agent Decision → Output → Clinical Record → Audit Trail
Test Evidence Repository:
Unit tests for individual agent behaviors
Integration tests for multi-agent workflows
User acceptance tests with clinical stakeholders
Compliance validation with regulatory experts
Continuous Compliance Philosophy:
Compliance isn't a gate review — it's embedded in daily development
Every prompt change gets compliance impact assessment
Every agent behavior gets documented with clinical rationale
Every output gets structured for regulatory inspection
The goal isn't "compliance-ready." The goal is "audit-confident" — where our system architecture itself generates the evidence regulators need.
Section 7: Flow State Reality (Founder Operator Lens)
Early mornings: Architecture diagrams on whiteboards, coffee getting cold while I map agent interaction patterns.
Late nights: Prompt tuning sessions, testing edge cases that clinical teams will definitely hit in production.
Sprint boards: Living documents that capture the orchestration between product development, clinical validation, and regulatory alignment.
Architecture maps: Visual representations of how AI agents, human workflows, and compliance requirements all fit together.
Systems thinking inside a clinical, regulated world: Every technical decision cascades through clinical workflows, regulatory requirements, change management implications, and operational support needs.
This is what it feels like to build at the intersection of AI innovation and clinical operations reality. The complexity is real. The regulatory requirements are non-negotiable. The patient impact is immediate.
And somehow, in the middle of this controlled chaos, there's flow state — where all the pieces start connecting, and you can see how to make "impossible" problems actually solvable.
What This Means for You
If you're a CRO or Sponsor: This is how AI actually gets implemented in clinical operations — not as magic, but as disciplined product development that respects clinical reality.
If you're a PI or Site: This is what it looks like when technology builders actually understand your workflow constraints and regulatory requirements.
If you're an Investor: This is how HealthTech companies successfully navigate the valley between "AI demo" and "scalable clinical operations."
If you're Talent: This is what it feels like to build products that actually move the needle in healthcare — where technical excellence meets clinical impact.
Ready to activate faster?
👉 Clinical Teams: DM me to discuss your activation bottlenecks
👉 Investors: Let's talk about regulated AI that actually works
👉 Builders: Join us in solving hard problems that matter
The future of clinical trials isn't just about better technology.
It's about better orchestration of people, process, and AI working together.
Let's build it.
Tags: #ClinicalTrials #AI #ProductOwner #ProductOps #PMO #OCM #HealthTech #AgenticAI #Compliance #GxP #Execution